NITROGEN (N2): BASIS OF LIFE
Nitrogen is used as a shielding gas in welding and to transport flammable substances. It serves as a propellant and as a filling gas for aircraft tires. Other application areas include refrigerator recycling, the cold grinding of plastics, and the chemical synthesis of nitrogen compounds on an industrial scale – in the production of active substances, for example.
As an essential component of amino acids, nitrogen is a fundamental building block of all life. Without the element with the symbol N, there would be no metabolism, no protein and no DNA – neither in plants nor in animals or humans. Nitrogen constitutes nearly two kilograms of the weight of a 70-kilogram adult.
Ninety-nine percent of all nitrogen on Earth is in the air. However, only a few plants from the bean family (legume family) can, with the aid of bacteria, absorb nitrogen directly from the atmosphere. All others require the solid nitrogen compounds contained in arable soil and consumed by the plants. That’s why over 80 percent of the world’s nitrogen production – some 40 million tons per year – is used just to produce chemical fertilizers.
Pure nitrogen is used for many purposes, including as a filling gas for aircraft tires, so they do not catch on fire from the heat generated during takeoff and landing. The gas serves as a packaging gas or a propellant, such as for whipped cream or in beverage dispensing systems that need especially high tap pressure.
Liquid nitrogen is used in cryotechnology as a cooling medium – for food storage, for example, or flash freezing. Other application areas for liquid nitrogen include concrete cooling and soil freezing in construction work as well as cryosurgery. The best known example of the latter is as a treatment to “freeze” warts off.
In German, nitrogen gets its name – Stickstoff – from its characteristic ability to smother both flames and living creatures. The scientific name nitrogenium derives from the Greek word for saltpeter (“nitros”), from which nitrogen was extracted before the invention of air separation.
Occurrence:
With about 78 %, it is the single largest component of air; its mass percent of the Earth’s four spheres combined is 0.03 %
Chemical properties:
The gas has a neutral odor and taste and condenses to a colorless liquid. Nitrogen is extremely inert; it is barely soluble in water and nonflammable. It is the third most electronegative element after fluorine and oxygen.

CRYO-SAUNA
Ice cold well-being

SUPERCONDUCTING POWER CABLES
Near zero resistance

ARTIFICIAL INSEMINATION
Fulfilling the desire to have children

FLASH FREEZING OF ICE CREAM
Crispy cold

WINE PRODUCTION
Bottling the bouquet

GASES FOR CRIME-FIGHTING
Gases for crime-fighting

A STRONG HEART FOR COMPUTERS, CELL PHONES, ETC.
Reliable computer brains

SUPERCONDUCTIVITY
Cryogenic high voltage
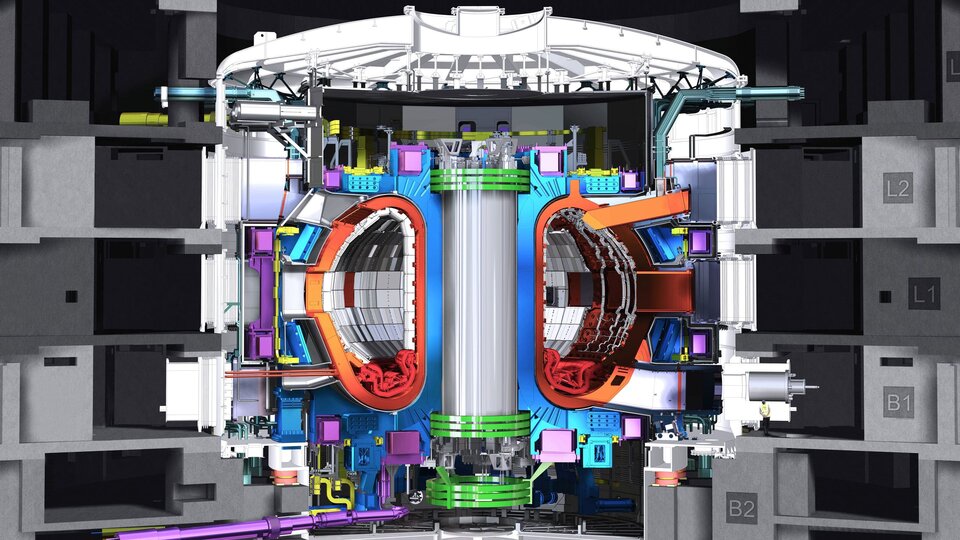
NITROGEN FOR ADVANCED TECHNOLOGY
Cooling nuclear fusion

TWO-FOLD NITROGEN SUPPLY FOR HOUSEHOLD APPLIANCES
Two-fold nitrogen supply for household appliances

SHRINK FITTING – PURE PHYSICS FOR A SECURE GRIP
Shrink fitting – pure physics for a secure grip

KEEPING STREETCARS CLEAN WITH DRY ICE BLASTING
Keeping streetcars clean with dry ice blasting

SAFE DRILLING INTO ROCK HARD SOIL
Rock solid safety

BLOOD PLASMA: RAW MATERIAL WITH HEALING POWER
Blood plasma: Raw material with healing power

PET BOTTLES
Form for fluids
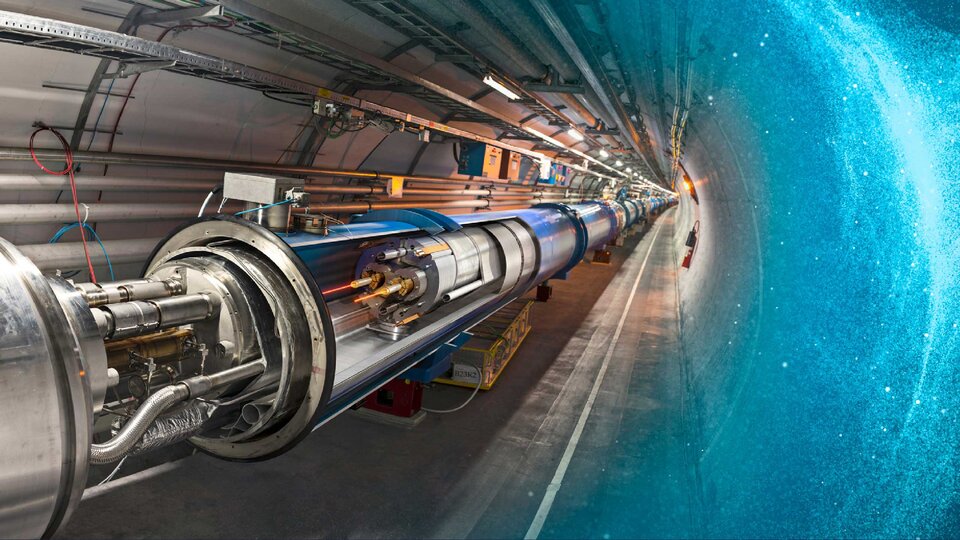
PARTICLE ACCELERATOR
Cooling for magnets

LIQUEFIED GASES FOR THE PRODUCTION OF TOUCH PANELS
Sensitive surfaces

SO (NON) HAZARDOUS!
So (non) hazardous!

INDISPENSABLE IN LABS AND OPERATING THEATERS
Indispensable in labs and operating theaters

“DUOCONDEX” PREVENTS TONS OF POLLUTION FROM HARMING THE ENVIRONMENT
Pollutants recovered

UPHOLSTERED FURNITURE
Sitting more comfortably

FREEZING ENABLES EFFICIENT PVC RECYCLING
PVC recycling

TRAVELING IN COMFORT AND SAFETY
Traveling in comfort and safety

TURNING GARDEN HOSES INTO GARDEN HOSES
Recycling garden hoses

PLAYFUL RECYCLING
Playful recycling

EFFICIENT CLARITY
Efficient clarity

CRYOGENIC GASES FOR FOREST BERRIES AND MUSHROOMS
Cryogenic gases for forest berries and mushrooms

LIQUEFIED GASES FOR IPHONE PRODUCTION
Perfectly connected

NITROGEN COOLS RECYCLING MILL
Nitrogen cools recycling mill

MUSICAL INSTRUMENTS
Sounds good!
NITROGEN AS SHIELDING GAS OPTIMIZES PRINTED CIRCUIT BOARD ASSEMBLY PROCESS
Two birds with one stone

CHEESE PROCESSING
Nachhaltiger genießen

GASES FOR MANUFACTURING UV DISINFECTION LAMPS
Gentle germ-killers

BAKING CARBON FIBER TO SAVE JET FUEL
Baking carbon fiber to save jet fuel

PACKAGING OF SNACKS
Nuts to be savored

WELL-PACKAGED FRESHNESS
Packaged freshness

ICE-COLD TECHNOLOGY FOR BLOOD AND BLOOD PRODUCTS
Ice-cold technology for blood and blood products

NITROGEN AND HELIUM FOR CUTTING-EDGE RESEARCH
Nitrogen and helium for cutting-edge research

POTATO PROCESSING
Just like freshly cooked food

E-BIKES
Reliable e-bike drives

DIVING
Safety at great depths

RUBBER DEFLASHING
Smooth edges

CRYOGENIC IS EXTRA-SAFE
Cryogenic is extra-safe

THIN-FILM SOLAR CELLS
Adaptable energy production

HVAC TECHNOLOGY
Airy by design

